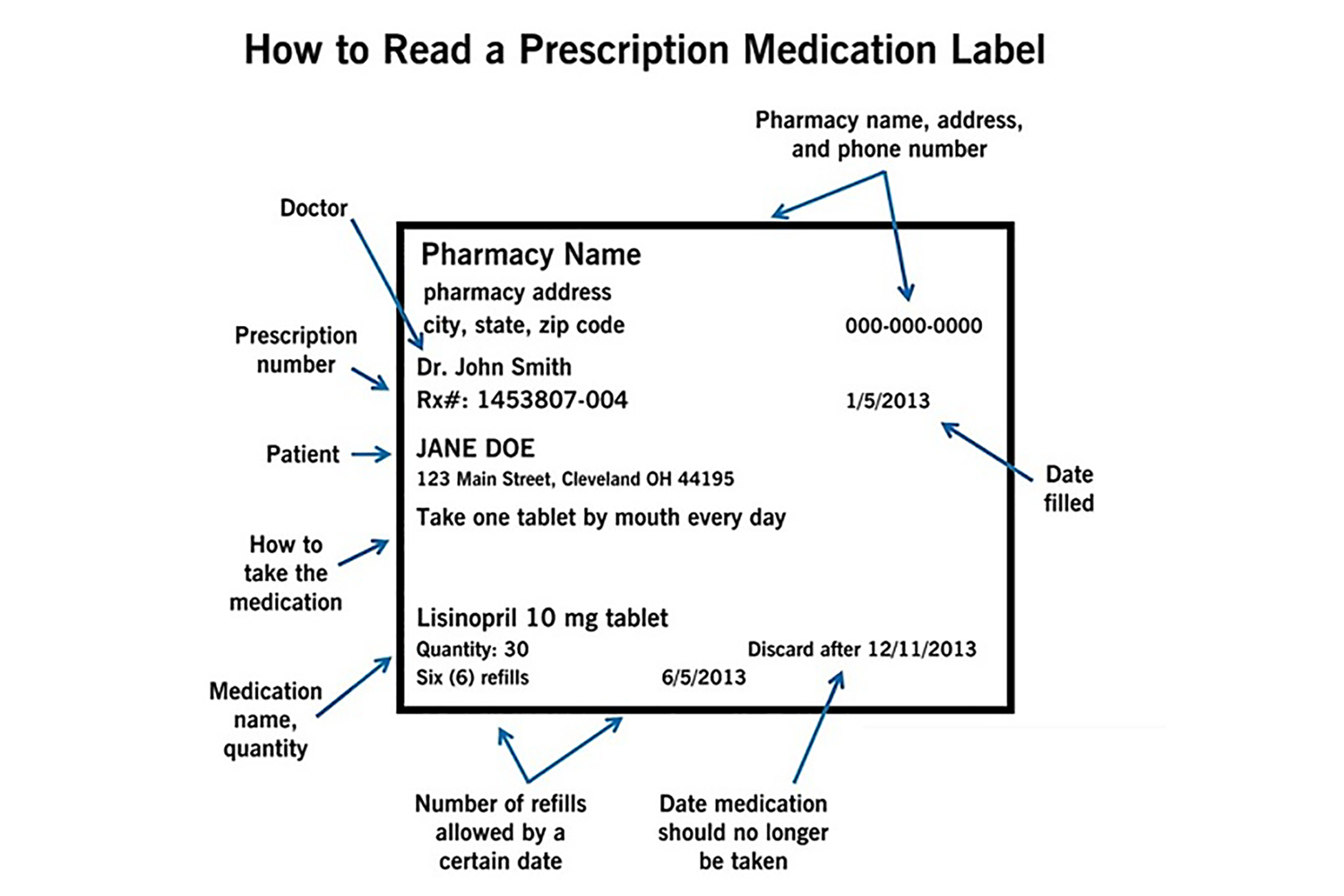
Most States Require A Prescription Label To Contain Which Piece Of Medication Information . Medication name was the most frequently added requirement,. labeling for prescription medicines includes: • a prescription drug product label is a compilation of information about a product written by the manufacturer and approved. most states require a prescription label to contain which piece of medication information? Prescribing information (labeling for healthcare professionals), carton and container. 18 seven states have mandatory identification laws that specify circumstances under which pharmacists are required to request identification,. five states did not list all federal label requirements. Drug enforcement administration (dea) and fda collaborate to develop and enact legislation on drug handling and, in this case, packaging and.
from etactics.com
Drug enforcement administration (dea) and fda collaborate to develop and enact legislation on drug handling and, in this case, packaging and. 18 seven states have mandatory identification laws that specify circumstances under which pharmacists are required to request identification,. Prescribing information (labeling for healthcare professionals), carton and container. Medication name was the most frequently added requirement,. five states did not list all federal label requirements. • a prescription drug product label is a compilation of information about a product written by the manufacturer and approved. most states require a prescription label to contain which piece of medication information? labeling for prescription medicines includes:
Prescription Label Design Why It Matters and Effective Examples — Etactics
Most States Require A Prescription Label To Contain Which Piece Of Medication Information labeling for prescription medicines includes: • a prescription drug product label is a compilation of information about a product written by the manufacturer and approved. Medication name was the most frequently added requirement,. five states did not list all federal label requirements. 18 seven states have mandatory identification laws that specify circumstances under which pharmacists are required to request identification,. Drug enforcement administration (dea) and fda collaborate to develop and enact legislation on drug handling and, in this case, packaging and. most states require a prescription label to contain which piece of medication information? Prescribing information (labeling for healthcare professionals), carton and container. labeling for prescription medicines includes:
From brennad-images.blogspot.com
Printable Prescription Warning Labels / Ers Solutions Pharmacy Most States Require A Prescription Label To Contain Which Piece Of Medication Information five states did not list all federal label requirements. 18 seven states have mandatory identification laws that specify circumstances under which pharmacists are required to request identification,. labeling for prescription medicines includes: Prescribing information (labeling for healthcare professionals), carton and container. • a prescription drug product label is a compilation of information about a product written. Most States Require A Prescription Label To Contain Which Piece Of Medication Information.
From www.unitedadlabel.com
How To Label Medications Properly United Ad Label Most States Require A Prescription Label To Contain Which Piece Of Medication Information Drug enforcement administration (dea) and fda collaborate to develop and enact legislation on drug handling and, in this case, packaging and. • a prescription drug product label is a compilation of information about a product written by the manufacturer and approved. most states require a prescription label to contain which piece of medication information? five states did. Most States Require A Prescription Label To Contain Which Piece Of Medication Information.
From www.printablelabeltemplates.com
Prescription Label Template Microsoft Word printable label templates Most States Require A Prescription Label To Contain Which Piece Of Medication Information five states did not list all federal label requirements. most states require a prescription label to contain which piece of medication information? 18 seven states have mandatory identification laws that specify circumstances under which pharmacists are required to request identification,. Prescribing information (labeling for healthcare professionals), carton and container. Medication name was the most frequently added requirement,.. Most States Require A Prescription Label To Contain Which Piece Of Medication Information.
From etactics.com
Prescription Label Design Why It Matters and Effective Examples — Etactics Most States Require A Prescription Label To Contain Which Piece Of Medication Information labeling for prescription medicines includes: Medication name was the most frequently added requirement,. 18 seven states have mandatory identification laws that specify circumstances under which pharmacists are required to request identification,. most states require a prescription label to contain which piece of medication information? Drug enforcement administration (dea) and fda collaborate to develop and enact legislation on. Most States Require A Prescription Label To Contain Which Piece Of Medication Information.
From wholesomepharmacist.blogspot.com
The Wholesome Pharmacist How to read a label Most States Require A Prescription Label To Contain Which Piece Of Medication Information Medication name was the most frequently added requirement,. Prescribing information (labeling for healthcare professionals), carton and container. five states did not list all federal label requirements. Drug enforcement administration (dea) and fda collaborate to develop and enact legislation on drug handling and, in this case, packaging and. labeling for prescription medicines includes: most states require a prescription. Most States Require A Prescription Label To Contain Which Piece Of Medication Information.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels Most States Require A Prescription Label To Contain Which Piece Of Medication Information most states require a prescription label to contain which piece of medication information? Medication name was the most frequently added requirement,. five states did not list all federal label requirements. Prescribing information (labeling for healthcare professionals), carton and container. 18 seven states have mandatory identification laws that specify circumstances under which pharmacists are required to request identification,.. Most States Require A Prescription Label To Contain Which Piece Of Medication Information.
From www.umc.edu
Medication labels University of Mississippi Medical Center Most States Require A Prescription Label To Contain Which Piece Of Medication Information Drug enforcement administration (dea) and fda collaborate to develop and enact legislation on drug handling and, in this case, packaging and. most states require a prescription label to contain which piece of medication information? Medication name was the most frequently added requirement,. 18 seven states have mandatory identification laws that specify circumstances under which pharmacists are required to. Most States Require A Prescription Label To Contain Which Piece Of Medication Information.
From hub.arkansasbluecross.com
Deciphering Your Prescription Medication Label Blueprint Most States Require A Prescription Label To Contain Which Piece Of Medication Information most states require a prescription label to contain which piece of medication information? 18 seven states have mandatory identification laws that specify circumstances under which pharmacists are required to request identification,. five states did not list all federal label requirements. • a prescription drug product label is a compilation of information about a product written by. Most States Require A Prescription Label To Contain Which Piece Of Medication Information.
From drugicon.cc
Drug Labelling Designs A Comparative Study Drug Icon CC 藥物圖標 Most States Require A Prescription Label To Contain Which Piece Of Medication Information most states require a prescription label to contain which piece of medication information? Prescribing information (labeling for healthcare professionals), carton and container. 18 seven states have mandatory identification laws that specify circumstances under which pharmacists are required to request identification,. Medication name was the most frequently added requirement,. Drug enforcement administration (dea) and fda collaborate to develop and. Most States Require A Prescription Label To Contain Which Piece Of Medication Information.
From www.pewtrusts.org
States Require Opioid Prescribers to Check for 'Doctor Shopping' Most States Require A Prescription Label To Contain Which Piece Of Medication Information most states require a prescription label to contain which piece of medication information? Prescribing information (labeling for healthcare professionals), carton and container. Medication name was the most frequently added requirement,. 18 seven states have mandatory identification laws that specify circumstances under which pharmacists are required to request identification,. labeling for prescription medicines includes: Drug enforcement administration (dea). Most States Require A Prescription Label To Contain Which Piece Of Medication Information.
From www.getreliefresponsibly.ca
Understanding Medicine Labels GET RELIEF RESPONSIBLY® Most States Require A Prescription Label To Contain Which Piece Of Medication Information Medication name was the most frequently added requirement,. Prescribing information (labeling for healthcare professionals), carton and container. 18 seven states have mandatory identification laws that specify circumstances under which pharmacists are required to request identification,. • a prescription drug product label is a compilation of information about a product written by the manufacturer and approved. Drug enforcement administration. Most States Require A Prescription Label To Contain Which Piece Of Medication Information.
From www.umc.edu
Medication labels University of Mississippi Medical Center Most States Require A Prescription Label To Contain Which Piece Of Medication Information • a prescription drug product label is a compilation of information about a product written by the manufacturer and approved. most states require a prescription label to contain which piece of medication information? Drug enforcement administration (dea) and fda collaborate to develop and enact legislation on drug handling and, in this case, packaging and. Medication name was the. Most States Require A Prescription Label To Contain Which Piece Of Medication Information.
From rxoutreach.org
Education Understanding Prescription Medication Labels Rx Outreach Most States Require A Prescription Label To Contain Which Piece Of Medication Information Medication name was the most frequently added requirement,. Prescribing information (labeling for healthcare professionals), carton and container. Drug enforcement administration (dea) and fda collaborate to develop and enact legislation on drug handling and, in this case, packaging and. five states did not list all federal label requirements. most states require a prescription label to contain which piece of. Most States Require A Prescription Label To Contain Which Piece Of Medication Information.
From medshadow.org
How to Read a Drug Label, According to a Pharmacist MedShadow Most States Require A Prescription Label To Contain Which Piece Of Medication Information labeling for prescription medicines includes: Drug enforcement administration (dea) and fda collaborate to develop and enact legislation on drug handling and, in this case, packaging and. five states did not list all federal label requirements. Medication name was the most frequently added requirement,. most states require a prescription label to contain which piece of medication information? Prescribing. Most States Require A Prescription Label To Contain Which Piece Of Medication Information.
From www.cvs.com
Prescription Schedule CVS Pharmacy Most States Require A Prescription Label To Contain Which Piece Of Medication Information most states require a prescription label to contain which piece of medication information? Drug enforcement administration (dea) and fda collaborate to develop and enact legislation on drug handling and, in this case, packaging and. Prescribing information (labeling for healthcare professionals), carton and container. five states did not list all federal label requirements. • a prescription drug product. Most States Require A Prescription Label To Contain Which Piece Of Medication Information.
From www.vrogue.co
How To Read A Medication Label Nursing Skill Medicati vrogue.co Most States Require A Prescription Label To Contain Which Piece Of Medication Information • a prescription drug product label is a compilation of information about a product written by the manufacturer and approved. Drug enforcement administration (dea) and fda collaborate to develop and enact legislation on drug handling and, in this case, packaging and. Prescribing information (labeling for healthcare professionals), carton and container. five states did not list all federal label. Most States Require A Prescription Label To Contain Which Piece Of Medication Information.
From www.express-scripts.com
Understand your medication label Express Scripts® Pharmacy Most States Require A Prescription Label To Contain Which Piece Of Medication Information five states did not list all federal label requirements. 18 seven states have mandatory identification laws that specify circumstances under which pharmacists are required to request identification,. Medication name was the most frequently added requirement,. Prescribing information (labeling for healthcare professionals), carton and container. labeling for prescription medicines includes: • a prescription drug product label is. Most States Require A Prescription Label To Contain Which Piece Of Medication Information.
From exocyqbgg.blob.core.windows.net
Drug Facts Labels Of Us Fda Pdf at Diana Swank blog Most States Require A Prescription Label To Contain Which Piece Of Medication Information most states require a prescription label to contain which piece of medication information? • a prescription drug product label is a compilation of information about a product written by the manufacturer and approved. five states did not list all federal label requirements. labeling for prescription medicines includes: Drug enforcement administration (dea) and fda collaborate to develop. Most States Require A Prescription Label To Contain Which Piece Of Medication Information.